Master Regulatory Processes with Confidence!
- A holistic overview of marketing authorization, variation, and renewal processes
- Content based on up-to-date legislation and Ministry practices
- Real-life cases and a practice-oriented approach
- Knowledge to manage processes more accurately, in a controlled and effective manner
Human Medicinal Products Variation Application Training
Make a difference with correct classification in variation procedures.
Manage your applications in a more controlled and efficient way.
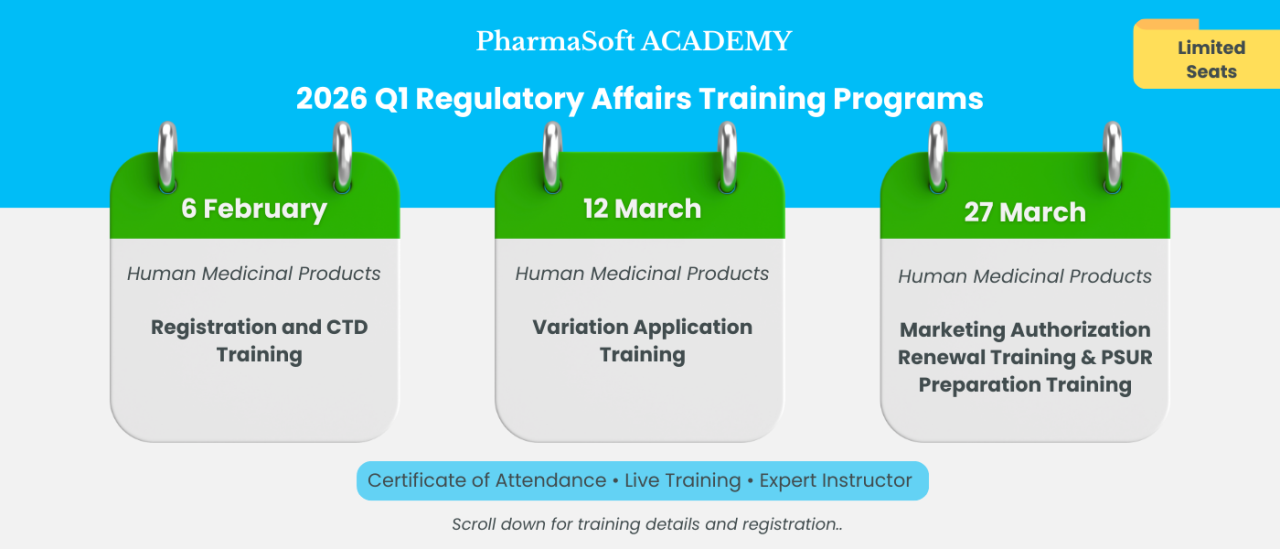
📅 Training Date: March 12, 2026
🕘 Time: 09:00 – 12:00
💻 Format: Live Online Training
📜 Certificate: Certificate of Participation
💰 Fee: 4,500 TRY (VAT included)
Training Program
Session I (09:15 – 10:15)
Overview of the Regulation and Guideline on Variations for Human Medicinal Products
Session II (10:30 – 11:30)
Practical Examples of Variation Applications
Session III (11:30 – 12:00)
Common Mistakes & Q&A
Trainer
Burcu Koyuncu, an industry professional with 17 years of experience in regulatory affairs
🎯 Key Learning Outcomes
- Correctly distinguish variation types (Type IA, Type IB, Type II, Grouping)
- Accurately assess changes within the scope of variations
- Perform variation classification in line with legislation and Ministry practices
- Correctly and completely identify CTD dossier sections to be updated
- Recognize common variation errors and reasons for authority feedback
- Reduce time and cost loss through correct classification
- Develop variation strategies for faster and smoother approvals
- Manage variation applications end-to-end in a more controlled manner
For detailed information: atelier@pharmasoft.com.tr
Limited seats available.
Human Medicinal Products Marketing Authorization Renewal and PSUR Preparation Training
Confidently manage marketing authorization renewal and PSUR processes.
Reduce the risk of errors and align with Ministry expectations.
📅 Training Date: 27 March 2026
🕘 Time: 09:00 – 14:00
💻 Format: Live Online Training
📜 Certificate: Certificate of Participation
💰 Fee: 5,500 TRY (VAT included)
Trainers
Burcu Koyuncu, an industry professional with 17 years of experience in regulatory affairs.
Rabia Ayşe Çetin, RPh, Co-Founder and General Manager of PharmaSoft, with over 25 years of experience in pharmacovigilance and drug safety.
Training Program
Session I (09:15 – 10:00)
Marketing Authorisation Renewal Procedures, SmPC/PIL Updates, Quality Overall Summary
Session II (10:15 – 11:15)
Preparation of the PSUR (Periodic Benefit-Risk Evaluation Report)
Session III (11:15 – 12:00)
Marketing Authorisation Renewal Application
Session IV (13:00 – 13:30)
Frequently Asked Questions and Key Points to Consider
Session V (13:30 – 14:00)
Q&A
🎯 Key Learning Outcomes
- Learn regulatory requirements and application steps for marketing authorization renewal
- Understand the scope, timing, and content of PSUR reports
- Plan marketing authorization renewal and PSUR processes in a harmonized manner
- Reduce the risk of incomplete or incorrect dossier submissions
- Develop timelines and strategies aligned with Ministry expectations
- Manage processes in a faster, more controlled, and sustainable way
- Identify common practical pitfalls and effective solution approaches
For detailed information: atelier@pharmasoft.com.tr
Limited seats available.
OUR PAST TRAININGS
Online CTD Dossier Preparation and Marketing Authorization Processes Training
Due to popular demand, back again! 🎯
In response to increasing participant interest, the Marketing Authorization Training, organized for the third time, returns with an updated date.
📅 Training Date: 6 February 2026
🕘 Time: 09:00 – 15:30
💻 Format: Live Online Training
📜 Certificate: Certificate of Participation
💰 Fee: 5,500 TRY (VAT included)
Trainer
Burcu Koyuncu, an industry professional with 17 years of experience in regulatory affairs.
Training Program
Session I (09:15 – 10:15)
- General Overview of Marketing Authorization
- Current Legislation
- Marketing Authorization of Human Medicinal Products
Session II (10:30 – 12:00)
- Marketing Authorization of Human Medicinal Products (Continued)
Session III (13:00 – 14:00)
- General Information on CTD Dossiers
- Preparation and Organization of the CTD Dossier
Session IV (14:15 – 15:00)
- eCTD Submission
Q&A (15:15 – 15:30)
🎯 Key Takeaways
- In-depth understanding of marketing authorization processes and current legislation
- Ability to structure CTD dossiers correctly
- Practical experience in eCTD submission steps
- Prevention of common mistakes in marketing authorization dossiers
Regulatory Affairs
Training
- OBJECTIVE
- CONTENT
- ASSESSMENT AND EVALUATION
- CERTIFICATE OF PARTICIPATION



